NAFDAC Alerts Nigerians On The Sale Of Counterfeit Tandak Injection In Nigeria
Posted by Amarachi on Thu 11th Apr, 2024 - tori.ng
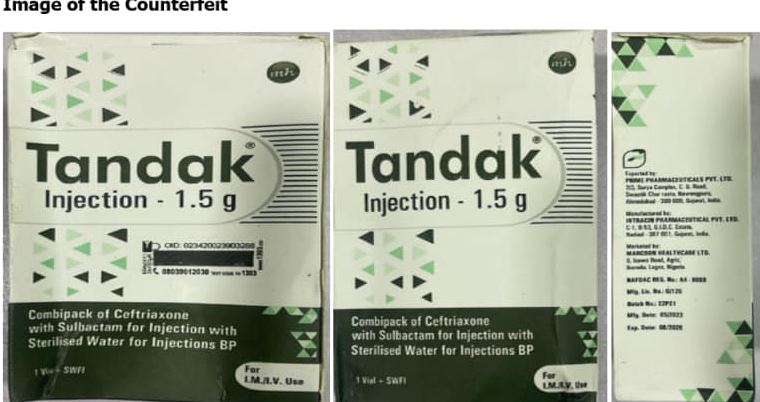
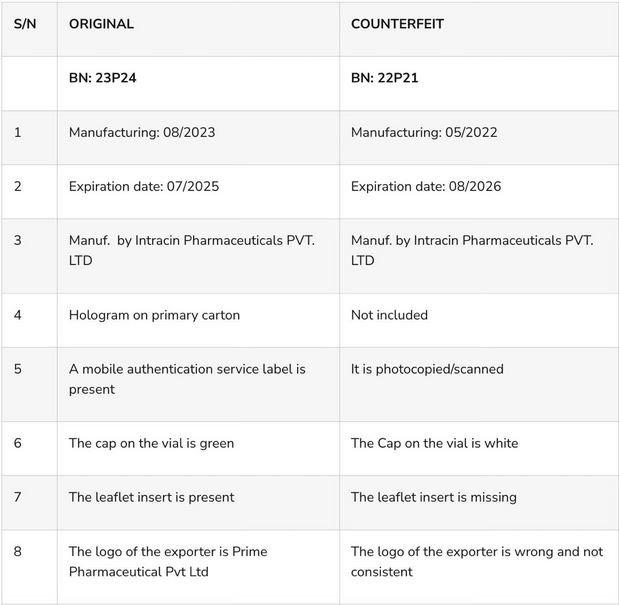
The National Drug Law Enforcement Agency (NAFDAC) has raised an alrm about the sale of counterfeit TANDAK injection 1.5g powder and water for injection, manufactured by Intracin Pharmaceuticals PVT. LTD C-1, B-53, G.I.D.C Estate, Nadiad- 387001, Gujarat, India.
A statement released by the agency on Wednesday, April 10, says the counterfeit product was discovered in Gombe State, Nigeria, and reported to the Agency by Marcson Healthcare Ltd. - the Marketing Authorisation Holder (MAH).
‘’Tandak® injection of 1.5g powder is a co-formulation of Ceftriaxone 1000mg and Sulbactam 500mg. It is prescribed for use in the treatment of various types of bacterial infections. It fights against micro organisms by preventing their growth, and further spread of the infection. Ceftriaxone+Sulbactam 1000mg/500mg Injection should only be administered under the supervision of a healthcare professional''. the statement read
The illegal marketing of counterfeit medicines poses a risk to the health of people, since by not complying with the regulatory provisions, the safety, quality, and efficacy of the products are not guaranteed.
NAFDAC has directed all its Zonal Directors and State Coordinators to carry out surveillance and mop up the counterfeit products within the Zones and States.
Healthcare professionals and consumers are advised to report any suspicion of the sale of substandard and falsified medicines or medical devices to the nearest NAFDAC office, call 0800-162-3322 or send an email to [email protected].